What is Vigabatrin?
Vigabatrin is an antiepileptic drug primarily used to manage infantile spasms, spasms in tuberous sclerosis complex, and refractory complex partial seizures. This medication functions as an irreversible inhibitor of GABA transaminase, increasing GABA levels in the brain, which helps control seizure activity. Indications include infantile spasms and cases of epilepsy that do not respond to other treatments. Vigabatrin is associated with significant adverse effects, including visual field defects, which have led to an FDA-issued box warning. Due to these risks, routine visual monitoring is essential during treatment.
In addition to adverse effects, vigabatrin’s various pharmacokinetic features may necessitate dose adjustments in patients with certain conditions. Contraindications, drug interactions, and recommended monitoring are among the topics discussed in this activity. A collaborative, interprofessional healthcare team approach is critical to ensuring the safe and effective management of patients receiving vigabatrin. The team must adhere to evidence-based guidelines and adjust treatment as needed to mitigate adverse effects while maximizing seizure control.
Vigabatrin Indications
Vigabatrin was first synthesized in 1974 as a treatment for seizures. Clinical trials started in Europe in 1979 and the United States in 1980. These trials led to the approval of vigabatrin for the general public in the UK in 1989. Initially, the drug was most commonly prescribed for infantile spasms and refractory complex partial seizures.
However, officials raised concerns about its safety in 1997 despite its efficacy, citing the increased incidence of peripheral vision loss in patients receiving vigabatrin. After a series of studies, the FDA approved vigabatrin in 2009 for the treatment of infantile spasms as a single drug and refractory complex partial seizures as an additional drug to other anti-epileptic drugs.
Given the potential risks of visual loss, the approval comes with a supplemental “Risk Evaluation and Mitigation Strategy.” The International Tuberous Sclerosis Complex (TSC) Consensus Group recommends vigabatrin as the first-line therapy for treating infantile spasms associated with tuberous sclerosis complex.
A systematic review and meta-analysis found that vigabatrin is less effective than hormonal monotherapy (ACTH or steroids) for treating infantile epileptic spasms syndrome in patients with non-tuberous sclerosis complex etiologies. However, vigabatrin is more effective for infantile epileptic spasms syndrome in patients with tuberous sclerosis complex (TSC), making it the first-line medication for these patients.
FDA-Approved Indications
The FDA has approved vigabatrin for the treatment of infantile epileptic spasms syndrome and refractory complex partial seizures.
Off-Label Uses
A systematic review suggests that vigabatrin may reduce seizure frequency in adults with drug-resistant focal epilepsy. Vigabatrin is more effective for infantile epileptic spasms syndrome in patients with tuberous sclerosis complex (TSC), making it the first-line medication for these patients. Caution is advised when considering vigabatrin due to its risk-benefit considerations.
Vigabatrin FDA Label
Vigafyde (vigabatrin) FDA Label
Download the Vigafyde label below or visit: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=217684
Sabril (vigabatrin) FDA Label
Download the Sabril label below or visit: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022006
Vigabatrin Mechanism of Action
Vigabatrin is an irreversible inhibitor of gamma-amino-butyric acid transaminase (GABA-T), an enzyme that degrades GABA, an inhibitory neurotransmitter. Vigabatrin is structurally similar to GABA, with an extra vinyl group. This similarity allows vigabatrin to act as a substrate for GABA-T, freeing GABA in the synaptic cleft. The concentration of GABA increases in the brain, which aids in terminating seizure activity. In addition to inhibiting GABA-T, vigabatrin also prevents the neuronal uptake of GABA and stimulates its release into the synapse. Some studies have demonstrated that vigabatrin enhances the action of the inhibitory neurotransmitter glutamine, which researchers suggest enhances its anticonvulsant effect.
Pharmacokinetics
Absorption: Complete absorption occurs after oral administration. Peak plasma concentrations are achieved in approximately 1 hour.
Distribution: Vigabatrin does not bind to plasma proteins and is widely distributed. The drug exhibits negligible plasma protein binding.
Metabolism: Vigabatrin is a mild inducer of CYP2C9, undergoes minimal hepatic metabolism, and is primarily excreted unchanged through the kidneys.
Excretion: Approximately 95% of vigabatrin is excreted unchanged in the urine.
Vigabatrin Dosage Forms and Strengths
Vigabatrin is available in multiple dosage forms to meet various patient needs, including tablets, a ready-to-use liquid solution, and an oral powder for solution. Among these, the ready-to-use liquid solution is widely regarded as the most convenient option, especially for infants and younger children due to its ease of administration. Notably, Vigafyde is the only brand that provides vigabatrin in this highly practical, ready-to-use liquid form. Below is a detailed breakdown of the available dosage forms and their strengths:
Ready-to-Use Liquid Solution
- Concentration: 100 mg/mL (Vigafyde)
- Preparation: None
- Suitable for: Infants, younger children, and patients with dysphagia (difficulty swallowing)
Tablets
- Strength: 500 mg per tablet
- Preparation: None
- Suitable for: Adults and older children
Powder for Oral Solution
- Strength: 500 mg per sachet
- Preparation: Multiple step process to dissolve in 10 mL of water to yield a 50 mg/mL concentration
- Suitable for: Infants, younger children, and patients with dysphagia (difficulty swallowing)
Vigabatrin Brands
Vigabatrin is available as Vigafyde in a ready-to-use liquid dosage form.
Vigabatrin is available as Sabril in a tablet and powder for oral solution dosage form.
Benefits of Vigafyde (vigabatrin liquid) vs Sabril (vigabatrin powder)
If you are considering vigabatrin for your child, talk to your doctor about the risks and benefits of each formulation. Your doctor can help you decide which formulation is best for your child. The benefits of liquid vigabatrin over vigabatrin powder are highlighted below:
- Convenience: Vigafyde is ready to use, while the powder needs to be mixed with water before each dose. This can be more convenient for caregivers, especially when traveling or in situations where mixing the powder may be difficult.
- Accuracy: Studies have shown that caregivers are more likely to give the correct dose of vigabatrin when using the ready-to-use liquid formulation compared to the powder. This is because there is less room for error when mixing the liquid.
- Reduced risk of contamination: The powder formulation may be more susceptible to contamination, while the ready-to-use liquid is sterile and less likely to be contaminated.
It is important to note that both Vigafyde ready-to-use liquid and vigabatrin powder are effective in treating infantile spasms. The choice between the two formulations depends on individual needs and preferences.
Vigabatrin Stability
Vigabatrin is available in three formulations: tablets, a powder for oral solution, and a ready-to-use liquid solution, each with distinct stability profiles. According to the prescribing information, vigabatrin tablets remain stable until their expiration date when stored at controlled room temperature, offering long-term stability without the need for preparation. However, vigabatrin powder, once reconstituted with water, must be used immediately, with any unused portion discarded due to potential degradation or microbial growth in the absence of preservatives. In contrast, the vigabatrin liquid solution (Vigafyde) maintains stability for 90 days after the bottle is opened, providing a significant advantage over the powder in terms of shelf life post-opening. The liquid formulation’s 90-day post-opening stability reduces waste, simplifies storage, and supports consistent dosing for patients requiring ongoing treatment, making it more practical than the powder, which demands frequent preparation and immediate use.
Vigabatrin Side Effects and Adverse Events
The side effects and adverse events of vigabatrin differ by age and condition treated. Below is a detailed list derived from Sabril and Vigafyde labels and clinical data.
Common Side Effects
- Adults: Blurred vision, drowsiness, dizziness, coordination issues or unsteady walking, tremor, fatigue, weight gain (5%), tiredness (9.2%).
- Children (3–16 years): Weight gain, with side effects mirroring adults (e.g., blurred vision, drowsiness, dizziness).
- Infants (1 month–2 years): Drowsiness (potentially affecting feeding or causing irritability), bronchitis, ear infections, irritability.
- Gastrointestinal: Abdominal pain (1.6%), constipation (1.4%), vomiting (1.4%), nausea (1.4%), dyspepsia (<1%), increased appetite (<1%).
- General: Weakness (asthenia, 1.1%).
Serious Adverse Events
- Severe Allergic Reactions: Rare but severe, with symptoms like hives, breathing difficulty, or swelling of face/lips/tongue/throat, requiring urgent medical care.
- Permanent Vision Loss: Causes bilateral concentric visual field constriction (tunnel vision) in up to 30% of patients, potentially disabling. May impair central retina, reducing visual acuity. Risk grows with higher doses and prolonged use, with no safe dose. Damage is typically irreversible, occurring during or after treatment. Symptoms include difficulty seeing peripherally, tripping, bumping into objects, or clumsiness. Vision tests every 3 months are mandatory but cannot prevent damage.
- Peripheral Neuropathy: Numbness or tingling in feet or toes, which may persist after stopping treatment.
- Suicidal Thoughts and Behaviors: Elevated risk of suicidal ideation or actions, requiring monitoring for new or worsening mood changes. Immediate action (e.g., calling 911 or 988 Suicide & Crisis Lifeline) is critical for suicidal behavior.
- Anemia: Reduced red blood cell counts (6% vs. 2% placebo), leading to fatigue or weakness.
- Edema: Peripheral edema (2% vs. 1% placebo) and generalized edema (1% vs. 0% placebo) in adults; pediatric data limited but suggest similar rates.
- Intramyelinic Edema (IME): Brain swelling observed in infant autopsies; clinical relevance unclear.
- MRI Abnormalities: Abnormal brain MRI signals (increased T2, restricted diffusion) in infants, affecting thalamus, basal ganglia, brainstem, and cerebellum; typically resolves post-treatment, but significance is unknown.
- Withdrawal Seizures: Abrupt discontinuation may trigger status epilepticus (life-threatening, prolonged seizures). Gradual tapering is essential.
Latest Vigabatrin Research
Liquid Medication Dosing Errors: Comparison of a Ready-to-Use Vigabatrin Solution to Reconstituted Solutions of Vigabatrin Powder for Oral Solution
Abstract
Introduction
Vigabatrin (VGB) is intended for use by caregivers of infants (1 month to 2 years old) diagnosed with infantile spasms (IS). Commercially available vigabatrin powders require caregiver reconstitution prior to oral administration. This study compared the ability of caregivers to accurately provide a targeted dose of VIGAFYDE™ (vigabatrin) ready-to-use (RTU) oral solution (VGB-RTU solution) and SABRIL® (vigabatrin) powder for oral solution (vigabatrin powder) without instruction from a healthcare professional.
Methods
A crossover comparative usability study with 30 lay users (15 caregivers with vigabatrin powder experience and 15 oral-syringe/medication preparation naïve users) which required users to deliver a single dose of both VGB-RTU surrogate solution and vigabatrin powder to a sample collection bottle was performed. Doses were measured analytically with a primary endpoint to deliver doses within ± 10% of the target dose of 1125 mg.
Results
All 30 participants administered Vigafyde VGB-RTU solution doses within ± 5% of the target, while only 23/30 of the Sabril vigabatrin powder doses were within ± 10%. All naïve users delivered vigabatrin doses using VGB-RTU solution within ± 5% of the target; whereas only 13/15 delivered doses within ± 10% for vigabatrin powder. All experienced vigabatrin users delivered calculated vigabatrin doses using VGB-RTU solution within ± 3%; whereas only 10/15 delivered doses within ± 10% for vigabatrin powder. Users were equally able to accurately deliver the prescribed volumes of both products. Calculated doses of VGB-RTU solution (mg) were significantly less variable (p < 0.0001) and more accurate (p < 0.01) than doses of vigabatrin powder.
Sabril (vigabatrin powder) vs Vigafyde (vigabatrin RTU solution)
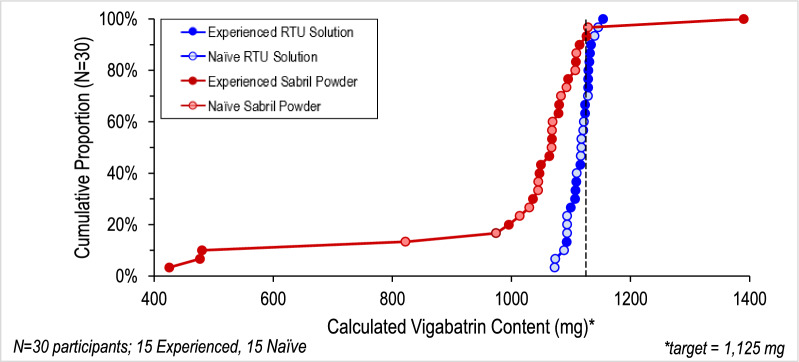
Conclusion
Caregivers delivered more accurate and less variable doses of the ready-to-use solution compared to solutions prepared from vigabatrin powders for oral solution. These differences were shown to be due to caregiver errors in reconstituting vigabatrin powders for oral solution.
Download the study below or visit: https://link.springer.com/article/10.1007/s12325-024-03089-0
The PREVeNT Trial
The PREVeNT study found that early vigabatrin treatment delayed the onset and reduced the overall prevalence of infantile spasms in TSC infants. However, the seizure prevention was not seen for other seizure types, including focal seizures, that are highly prevalent in this population. PREVeNT, similarly to EPISTOP, reported a reduced incidence of infantile spasms up to 24 months of age.
Download the study below or visit: https://pubmed.ncbi.nlm.nih.gov/37638552
EPISTOP Trial
Infantile spasms are seen in 50 to 70% of children with TSC, and are associated with both drug-resistance and intellectual disability. Importantly, in EPISTOP, none of the children who received preventive treatment developed infantile spasms throughout the 2-year course of the study, in contrast to 10 of 25 (40%) receiving conventional treatment.
Download the study below or visit: https://pmc.ncbi.nlm.nih.gov/articles/PMC7898885
Vigabatrin Risk Evaluation and Mitigation Strategy (REMS)
A REMS is a strategy to manage known or potential serious risks associated with a drug product, and is required by the Food and Drug Administration (FDA) to ensure the benefits of a drug outweigh its risks. The vigabatrin black box warning alerts healthcare providers and patients to the serious risk of permanent vision loss, specifically bilateral concentric visual field constriction, which can lead to tunnel vision or disability and may occur unpredictably during treatment. The REMS (Risk Evaluation and Mitigation Strategy) program is a mandatory safety protocol requiring prescriber certification, patient enrollment, and regular vision monitoring to ensure the drug’s benefits outweigh its risks. This program also restricts vigabatrin dispensing to certified pharmacies and mandates periodic vision assessments to manage and mitigate the risk of vision impairment.
Specifically, the current goals and purpose of the Vigabatrin REMS is to mitigate vision loss associated with vigabatrin by:
- Ensuring that healthcare providers are educated about the risk of vision loss, the need to counsel patients about the risk, and the need for periodic visual monitoring
- Ensuring that vigabatrin is dispensed only to patients with documentation that they are informed about the risk of vision loss associated with vigabatrin and the need for periodic visual monitoring
You can learn more about the Vigabatrin REMS here: https://www.vigabatrinrems.com/
Overview of Vigabatrin on the NIOSH List
Vigabatrin is included on the NIOSH List of Hazardous Drugs in Healthcare Settings (2024), categorized under miscellaneous anticonvulsants. It meets NIOSH criteria as a hazardous drug specifically due to its developmental and/or reproductive toxicity, which can disrupt fetal development (e.g., teratogenic effects) or impair fertility and the ability to conceive or carry a pregnancy to term. It is not classified as carcinogenic by NTP or IARC, and lacks manufacturer’s special handling information (MSHI). This classification indicates it poses risks primarily related to reproduction and development rather than other hazards like genotoxicity or organ toxicity at low doses.
Vigabatrin Exposure Risks
Occupational exposure to vigabatrin can lead to adverse reproductive and developmental effects, particularly for healthcare workers who are actively trying to conceive, pregnant, may become pregnant, or breastfeeding (as it may be excreted in breast milk). Risks are influenced by the drug’s toxicity, formulation, handling activities (e.g., compounding, administration, crushing), and routes of exposure such as skin absorption, inhalation of dust or aerosols, ingestion, or accidental injection. High-risk activities include manipulating uncoated tablets, reconstituting powders (dust generation), or handling liquids (splashes). Factors like dose, frequency of handling, and lack of controls amplify risks, potentially leading to surface contamination or inadvertent contact. Site-specific risk assessments are recommended to evaluate exposure potential.
Vigabatrin Proper Handling and Disposal
Handling should follow a hierarchy of controls: engineering (e.g., ventilated enclosures), administrative (e.g., training, SOPs), and PPE. Avoid crushing tablets or manipulating forms outside controlled environments; use dedicated equipment and clean surfaces after use. For disposal, classify waste as trace chemotherapy (e.g., empty containers, PPE) and incinerate at regulated facilities. Seal containers when three-fourths full, comply with RCRA and local regulations, and develop facility-specific procedures. Detailed guidance emphasizes using intact forms when possible and conducting risk assessments for activities like receiving, compounding, or administering.
Vigabatrin Personal Protective Equipment (PPE)
PPE varies by activity and formulation but includes chemotherapy-rated gloves (powder-free, changed every 30 minutes), disposable impervious gowns, and respiratory protection (e.g., N95) for inhalation risks. Eye/face protection is needed for splash potential, and hair/shoe covers for compounding. Double gloving is standard for higher-risk tasks; always inspect for defects and remove properly to avoid contamination.
Comparison of Vigabatrin Safety by Formulation
Based on the NIOSH guidelines, the safety of vigabatrin formulations for occupational handling is evaluated by exposure potential. The liquid oral solution (e.g., Vigafyde) presents the lowest exposure risk as it requires no reconstitution or crushing, minimizing dust and aerosols. Tablets have a medium risk, safer when intact but higher if crushed for dosing. The powder form poses the highest risk due to dust generation during mixing.
| Formulation | Exposure Risks | Handling Requirements | Relative Exposure Risk (for Occupational Exposure) |
|---|---|---|---|
| Liquid Oral Solution (e.g., Vigafyde, ready-to-use) | Low risk; splash/spill risks during pouring/administration (skin/eye contact); lower aerosol than powders or crushed tablets. No reconstitution needed. | Administer with care to avoid splashes. Recommended over powder mixing and tablet crushing. | Lowest exposure risk (safest overall, preferred to reduce exposure). |
| Tablets (e.g., Sabril tablets) | Moderate risk; low if intact (minimal dust/splash); high if crushed (dust, surface contamination). Often need manipulation for pediatric dosing. | Count/dispense intact in non-ventilated area; crush in ventilated enclosure (e.g., BSC) or pill pouch to contain aerosols. | Medium exposure risk (safer than powder but requires caution if manipulated). |
| Powder for Oral Solution (e.g., Sabril packets) | High risk; dust generation during mixing/reconstitution (inhalation, skin absorption risks). | Compound in ventilated enclosure (e.g., BSC/CACI); use closed-system devices if possible; avoid open handling. | Highest exposure risk (least safe due to inherent dust risks). |
Vigabatrin Frequently Asked Questions (FAQs)
What is the black box warning for vigabatrin?
The black box warning for vigabatrin addresses the risk of permanent vision loss, including bilateral concentric visual field constriction (“tunnel vision”) and potential reductions in visual acuity, which can affect 30% or more of patients overall. While this risk applies to all patients, it is less well-documented in infants (aged 1 month to 2 years) because assessing vision in this age group is challenging. Visual field testing is often unreliable in infants, and vision loss may not be detected until it becomes severe or until the child is older. The warning still applies, as the risk cannot be ruled out, but clinical data specific to infants is limited due to these diagnostic difficulties. Regular vision monitoring, as required by the Vigabatrin REMS Program, is critical, with eye exams recommended within 4 weeks of starting treatment, every 3 months during treatment, and 3–6 months after discontinuation. Parents should watch for signs like difficulty tracking objects or clumsiness and report these to their doctor immediately.
Is there a vigabatrin ready-to-use liquid?
Yes, a ready-to-use (RTU) vigabatrin oral solution is available under the brand name Vigafyde, approved by the FDA in June 2024. It is the first and only premixed vigabatrin liquid formulation, designed for infants (1 month to 2 years) with infantile spasms where benefits outweigh the risk of vision loss. Unlike vigabatrin powder (e.g., Sabril), which requires reconstitution with water before each dose, Vigafyde simplifies preparation by eliminating mixing, reducing dosing errors, and offering a 90-day shelf life after opening.
What does vigabatrin do to GABA?
Vigabatrin increases levels of gamma-aminobutyric acid (GABA) in the brain by irreversibly inhibiting GABA transaminase (GABA-T), the enzyme responsible for breaking down GABA. GABA is a neurotransmitter that inhibits nerve activity, helping to calm excessive electrical signaling in the brain that can lead to seizures. By blocking GABA-T, vigabatrin allows GABA to accumulate, enhancing its inhibitory effects and reducing seizure activity. This mechanism makes it effective for treating conditions like infantile spasms and refractory complex partial seizures.
Can you mix vigabatrin powder with other liquids?
The vigabatrin powder for oral solution should be mixed only with cold or room temperature water, as specified in the prescribing information. The contents of the packet (typically 500 mg) should be dissolved in 10 mL of water to ensure accurate dosing and proper absorption. Mixing with other liquids, such as juice, milk, or formula, is not recommended because it has not been studied and could affect the medication’s stability, absorption, or dosing accuracy. If a patient cannot take the powder for oral solution with water, please consult your healthcare provider or consider the ready-to-use vigabatrin formulation.
For the ready-to-use liquid formulation (Vigafyde), it is a premixed oral solution designed for direct administration and should not be mixed with other liquids unless explicitly advised by a healthcare provider.
Can vigabatrin tablets be crushed?
Vigabatrin tablets should not be crushed, chewed, or split. The tablets are designed to be swallowed whole with water to ensure proper delivery and absorption of the medication. Crushing or altering the tablet can affect its efficacy and may increase the risk of side effects or improper dosing.
For patients who cannot swallow tablets, such as infants or those with swallowing difficulties, vigabatrin is available as a powder for oral solution, which is mixed with water before administration. Additionally, a ready-to-use (RTU) liquid formulation, Vigafyde, is available. This premixed liquid eliminates the need for reconstitution and simplifies dosing.
Why is vigabatrin on the NIOSH Hazardous Drug List?
Vigabatrin is included on the NIOSH Hazardous Drug List due to its potential to cause developmental and reproductive harm, as evidenced by animal studies showing developmental abnormalities like cleft palate and embryofetal deaths in rabbits. The National Institute for Occupational Safety and Health (NIOSH) classifies drugs as hazardous if they exhibit characteristics such as teratogenicity or reproductive toxicity, necessitating strict handling precautions to protect healthcare workers and caregivers from occupational exposure that could lead to adverse health effects, particularly for those who are pregnant or trying to conceive.
To minimize exposure risks, healthcare workers and caregivers are better suited to use ready-to-use formulations of vigabatrin, such as Vigafyde, an oral solution that eliminates the need for crushing or dissolving tablets, which can release hazardous drug particles. Adhering to safety guidelines like those in USP <800> and NIOSH’s Managing Hazardous Drug Exposures is critical, but ready-to-use formulations further reduce direct contact with the drug, enhancing safety for those handling it, especially in healthcare settings or home care environments where patients or caregivers may be involved in administration.
Does vigabatrin cause weight gain?
Yes, vigabatrin can cause weight gain as a side effect, particularly in children aged 3 to 16 years, where it is listed as a common side effect in clinical studies. In adults, weight gain is less frequently reported but can still occur. The exact mechanism by which vigabatrin may lead to weight gain is not fully understood, but it may be related to its effects on the central nervous system or changes in appetite or metabolism.
What are the most common side effects of vigabatrin?
Common side effects include:
- Adults: Blurred vision, drowsiness, dizziness, tremor, fatigue, and problems with coordination.
- Children (3–16 years): Weight gain, along with side effects seen in adults.
- Infants: Sleepiness (which may affect feeding), irritability, bronchitis, and ear infections.
Contact your healthcare provider if side effects persist or worsen.
What are the signs of vigabatrin vision loss to watch for in infants?
Detecting vision loss in infants (aged 1 month to 2 years) is challenging, as visual field testing is often unreliable in this age group. Signs to watch for include:
- Difficulty Tracking Objects: Your infant may not follow moving objects or faces with their eyes as expected.
- Clumsiness or Uncoordinated Movements: Bumping into objects or difficulty grasping items may indicate peripheral vision issues.
- Unusual Eye Movements: Excessive blinking, squinting, or lack of response to visual stimuli could suggest problems.
- Irritability or Behavioral Changes: Vision changes may cause frustration or discomfort. Report these signs to your doctor immediately.
How can I help my child cope with vigabatrin side effects like drowsiness?
Drowsiness is a common side effect of vigabatrin, particularly in infants and children. To help your child cope:
- Adjust Daily Routines: Schedule doses around nap or bedtime to minimize disruption from drowsiness. Avoid activities requiring alertness (e.g., active play) shortly after dosing.
- Monitor Feeding: In infants, drowsiness may affect feeding. Offer smaller, more frequent feeds if irritability or sleepiness interferes. Consult a pediatrician if feeding issues persist.
- Create a Calm Environment: Reduce stimulation (e.g., loud noises, bright lights) to help your child rest comfortably.
- Report Persistent Issues: If drowsiness significantly impacts your child’s daily activities or worsens, contact your healthcare provider, as a dose adjustment or additional support may be needed.
Can you use vigabatrin for tuberous sclerosis complex (TSC)?
Yes, vigabatrin is used for tuberous sclerosis complex (TSC), particularly to treat infantile spasms, a common seizure type in TSC. It is highly effective, stopping spasms in up to 95% of infants, often within days, and is recommended as a first-line treatment. It may also be used preventively to delay seizure onset, though it shows mixed results for cognitive benefits and focal seizures. Side effects, especially visual field defects, require regular monitoring.
How much does vigabatrin cost?
Vigabatrin is available in various formulations under different brand names, with Wholesale Acquisition Costs (WAC) varying by product. Below is a table summarizing the WAC prices for select options. Note that these are list prices and actual costs to patients may differ significantly based on insurance coverage, commercial payors, Medicaid, Medicare, discounts, or patient assistance programs. Prices for drugs can fluctuate and vary by location and provider.
| Product | Formulation | Quantity | WAC Price | Cost per mg |
|---|---|---|---|---|
| Vigafyde | Oral Solution (100 mg/mL) | 150 mL bottle (15,000 mg total) | $3,555.27 | $0.237 |
| Sabril | Oral Packet | 500 mg, 50 sachets (25,000 mg total) | $10,091.04 | $0.404 |
| Sabril | Oral Tablet | 500 mg, 100 tablets (50,000 mg total) | $20,182.05 | $0.404 |
Vigafyde is cheaper on a mg-to-mg basis compared to Sabril and offers a better formulation as a ready-to-use, concentrated oral solution (100 mg/mL), which requires smaller volumes for dosing and is easier to administer, especially for infantile spasms in pediatric patients. There are generics available for vigabatrin, some of which are priced above Vigafyde depending on the specific manufacturer, formulation, and market factors. Additionally, some manufacturers, such as the one for Vigafyde, provide patient support services to help with access and affordability.
How do you mix vigabatrin powder?
As a parent mixing vigabatrin powder at home for a child with conditions like infantile spasms, recognize that the drug is on the NIOSH hazardous drugs list due to potential reproductive and developmental toxicity. While USP <800> standards are primarily designed for healthcare professionals in controlled settings with specialized equipment like biological safety cabinets and full PPE ensembles, parents can adapt its core principles to minimize exposure risks such as inhalation, skin contact, or accidental ingestion during home handling. This includes wearing disposable gloves (preferably chemotherapy-rated if available) and an N95 mask or equivalent for respiratory protection, working in a well-ventilated area away from food preparation or other family members, washing hands thoroughly before and after handling, and avoiding direct contact with the powder. Consult your child’s doctor or pharmacist for personalized guidance, and consider using the pre-mixed liquid formulation (e.g., Vigafyde) if available to avoid handling powder altogether. If you’re pregnant, planning pregnancy, or breastfeeding, take extra precautions as the drug may pose risks, and dispose of any waste (like used gloves or packets) as trace hazardous waste per local guidelines, such as sealing in a plastic bag before throwing away.
To mix the powder, start by gathering supplies: the prescribed number of 500 mg packets, clean cups (one clear for mixing), cold or room temperature water, oral syringes (3 mL and 10 mL), and scissors. Note that the powder should not be mixed with any other liquid—only water should be used, as mixing with juice, milk, or other beverages could affect the drug’s stability, absorption, or safety. Tap packets to settle powder, cut open, and empty into the mixing cup. Measure 10 mL water per packet using the 10 mL syringe (e.g., 20 mL for two packets), add to the powder, and stir until fully dissolved and clear. Immediately draw up the doctor’s specified dose with the appropriate syringe (adjust for air bubbles), administer slowly into the child’s cheek or via feeding tube if needed, and discard any leftover mixture—do not store or reuse. Give doses twice daily with or without food, but monitor for side effects like vision changes or sleepiness, and never stop abruptly without medical advice to avoid seizure risks. Clean equipment with soap and water, and store packets at room temperature out of reach.
What is Vigadrone?
Vigadrone is a generic version of the brand-name medication Sabril, available in both powder and tablet forms. It contains the active ingredient vigabatrin, which is used to treat certain types of seizures, such as infantile spasms and refractory complex partial seizures in adults and children. In addition to Vigadrone, other non-brand generic versions of vigabatrin are also available on the market. However, Vigafyde is the only ready-to-use vigabatrin oral solution currently available, and it has no generic equivalents.
Vigabatrin Dosing Calculator
Vigabatrin Dosing Calculator
Dosing Results
Note: This calculator is for informational purposes only. Consult a healthcare provider for personalized dosing and review the Vigabatrin REMS program for vision monitoring requirements.
Support Groups for Infantile Spasms
- Infantile Spasms Action Network (ISAN) – https://infantilespasms.org
- Epilepsy Foundation – https://www.epilepsy.com/programs/family-services
- Infantile Spasms Community Facebook – https://www.facebook.com/groups/infantilespasmscommunity
- Epilepsy Alliance America – https://epilepsyallianceamerica.org/programs-services/support-groups
- r/infantilespasms Reddit – https://www.reddit.com/r/infantilespasms
Support Groups for Tuberous Sclerosis Complex (TSC)
- Tuberous Sclerosis Alliance (TSC Alliance) – https://www.tscalliance.org/find-support/community-support-network
- Tuberous Sclerosis Association (TSA) – https://tuberous-sclerosis.org/
- TSC Alliance Tuberous Sclerosis Complex Facebook – https://www.facebook.com/groups/TS.Alliance.Online.Discussion.Group
- r/TuberousSclerosisComp Reddit – https://www.reddit.com/r/TuberousSclerosisComp/
Sources:
https://www.ncbi.nlm.nih.gov/books/NBK557579
https://link.springer.com/article/10.1007/s12325-024-03089-0
https://en.wikipedia.org/wiki/Vigabatrin
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a88ac1b4-e2c9-45c0-b321-4785902172e3
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8d3d6316-33ab-41e8-9485-4495c218be56
https://www.cdc.gov/niosh/docs/2025-103/default.html
Note: This is for informational purposes only. For medical advice or diagnosis, consult a professional. Consult a healthcare provider for personalized guidance and review the FDA-approved Medication Guide for full details.